

For simplicity, you might want to use the term “molecule” for both covalently and ionically bonded substances. Note: In an ionically bonded substance such as NaCl, the smallest ratio of positive and negative ions bonded together is called a “formula unit” rather than a “molecule.” Technically speaking, the term “molecule” refers to two or more atoms that are bonded together covalently, not ionically. Cup with salt from evaporated saltwater.Safetyīe sure you and the students wear properly fitting goggles. The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. Evaluationĭownload the student activity sheet, and distribute one per student when specified in the activity. Students will be able to explain the process of the formation of ions and ionic bonds.
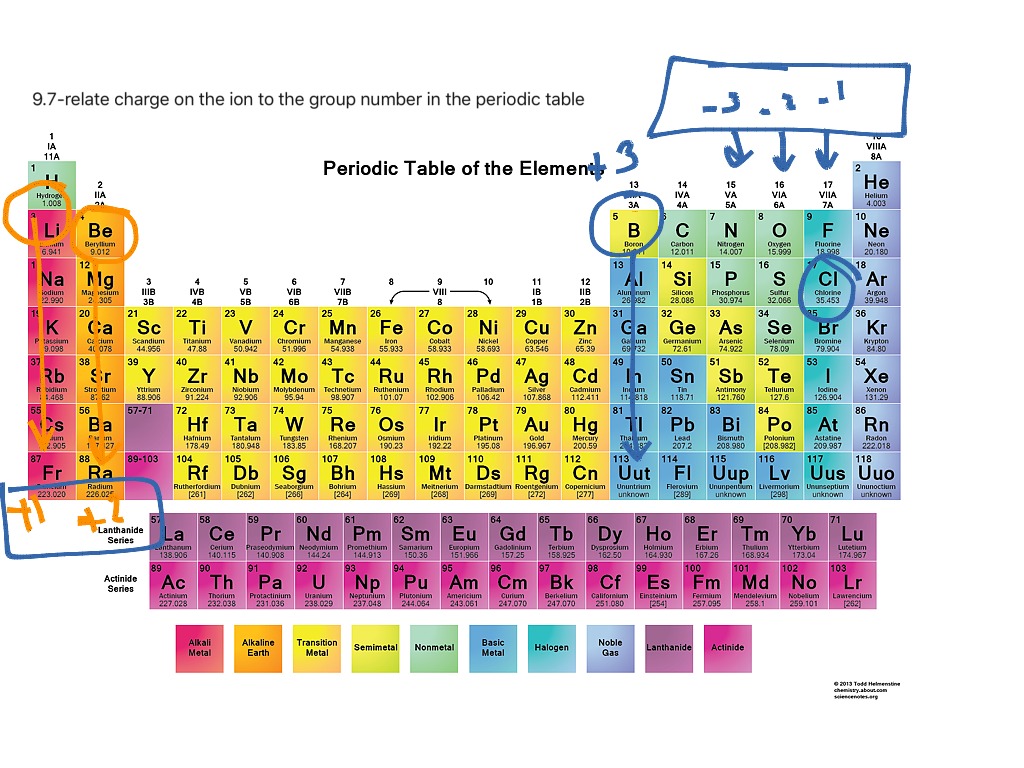
Students will use Styrofoam balls to make models of the ionic bonding in sodium chloride (salt). But in ionic bonding, electrons are transferred from one atom to the other and not shared like in covalent bonding. Students will see that both ionic and covalent bonding start with the attractions of protons and electrons between different atoms. Students will look at animations and make drawings of the ionic bonding of sodium chloride (NaCl). A positive and negative ion attract each other and form an ionic bond.The atom that gains an electron becomes a negative ion.The atom that loses an electron becomes a positive ion.When an atom loses or gains an electron, it is called an ion.The attractions between the protons and electrons of atoms can cause an electron to move completely from one atom to the other.


 0 kommentar(er)
0 kommentar(er)
